Stability and Reactivity Trends of Alkylidene Dihydropyridines
Matthew Puzhistky, Andrei Nikolaev, Ekadashi Pradhan, Tao Zeng, Dan Fishlock and Arturo Orellana*
2024, submitted.
Collaboration with F. Hoffmann - La Roche.
Mild and Catalytic Electrocyclization of Heptatrienyl Anions
Faizan Rasheed, Andrei Nikolaev, Anmol Dhesi, Tao Zeng, You Xuan Guo, Yarkali Krishna, Samira Komijani and
Arturo Orellana*
2024, submitted
The Electrocyclization of Heptatrienyl Anions
Samira Komijani and Arturo Orellana*
Synthesis 2024, 56, 701

Soft Fluorination of 4-Alkylpyridines
Faizan Rasheed, Jiaqi Shi, Tao Zeng, Yarkali Krishna, Dan Fishlock and Arturo Orellana*
Organic Letters 2023, 25, 8628
Collaboration with F. Hoffmann - La Roche.

Mild Installation of Piperidines on 4-Alkylpyridines
Jiaqi Shi, Faizan Rasheed, Dan Fishlock and Arturo Orellana*
Organic Letters 2023, 25, 4852.
Collaboration with F. Hoffmann - La Roche.
Highlighted in Organic Process Research and Development 2023, 27, 1535.
Jiabao Liu, Cigdem Sahin, Samar Ahmad, Lilia Magomedova, Minho Zhang, Zhengping Jia, Adam H. Metherel, Arturo Orellana, Gennady Poda, Richard P. Bazinet, Liliana Attisano, Carolyn L. Cummins, Hui Peng and Henry Krause*
Science Signalling 2022,15, 741.
Collaboration with Henry Krause - Donnelly Center for Cellular and Biomolecular Research, University of Toronto

Jiaqi Shi, Ashik A. Sayyad, DanFishlock and Arturo Orellana*
Organic Letters 2022, 24, 48.
Collaboration with F. Hoffmann - La Roche


Palladium-Catalyzed Mild Dehydrogenation of 4-Alkylpyridines
Nour Wasfy, Faizan Rasheed, Brian Doan, Dan Fishlock and Arturo Orellana*
ACS Catalysis 2021,11, 3251.
We disclose a very mild and general method to prepare 4-alkenylpyridines, which are useful building blocks in drug discovery.
Collaboration with F. Hoffmann - La Roche


Pyridylic Anions are Soft Nucleophiles in the Palladium-Catalyzed Allylation of Alkylpyridines
Nour Wasfy, Faizan Rasheed, Raphael Robidas, Isabelle Hunter, Jiaqi Shi, Brian Doan, Claude Y. Legault,* Dan Fishlock and Arturo Orellana*
Chemical Science 2021,12, 1503.
We develop an exceptionally mild method for the selective allylation of 4-alkylpyridines.
Collaboration with F. Hoffmann - La Roche.
highlighted in SYNFACTS 2021, 17, 293
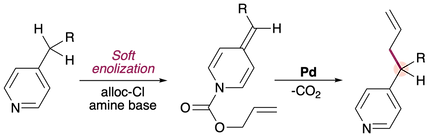

Enantioselective Synthesis of 7(S)-Hydroxydocosahexaenoic Acid,
a Possible Endogenous Ligand for PPAR-alpha
Minhao Zhang, Ashik A. Sayyad, Anmol Dhesi and Arturo Orellana*
The Journal of Organic Chemistry 2020, 85, 13621.
We report the first total synthesis of this biologically important natural product.


Andrei Nikolaev, Claude Y. Legault, Minhao Zhang and Arturo Orellana*
Organic Letters 2018, 20, 796-799.
We developed a method for direct alkylation of pyridines and related heterocycles using a well-defined silver catalyst. Along the way we uncovered the catalytic activity of in-situ formed silver-heterocycle complexes.

Convenient Access to meta-Substituted Phenols by Palladium-Catalyzed Suzuki Coupling and Oxidation
Zi Wang and Arturo Orellana*
Chemistry - A European Journal 2017, 23, 11445
We develop a new method for the synthesis of meta-substituted phenols by merging the Suzuki-Miyaura reaction and the palladium-catalyzed oxidation of cyclohexenones to phenols.
Chosen as a VIP contribution by the editorial board

Separating the Inflammatory and Diabetogenic Effects of Glucocorticoids Through LXR-beta Antagonism
Rucha Patel, Lilia Magovedova, Ricky Tsai, Stephane Angers, Arturo Orellana and Carolyn Cummins*
Endocrinology 2017, 158, 1034
Collaboration with Carolyn Cummins - Leslie Dan School of Pharmacy, University of Toronto.
A New Route to Phenols: Palladium-Catalyzed Cyclization and Oxidation of g,d-Unsaturated Ketones
Sadaf Samadi and Arturo Orellana*
ChemCatChem 2016, 8, 2472.
We describe a conceptually new method for the synthesis of substituted phenols that avoids many of the limitiations inherent in other routes.
Andrei Nikolaev and Arturo Orellana*
Organic Letters 2015, 17, 5796
We develop a practical route to acyl silanes. The substrates are easily prepared by addition of silylated acetylenes to aldehydes or ketones. A variety of acyl silanes are prepared in good yields.
Transition Metal-Catalyzed C-C and C-X Bond-Forming Reactions Using Cyclopropanols
Andrei Nikolaev and Arturo Orellana*
Synthesis 2016, 48, 1741
A comprehensive review of the literature from 2000 to 2016.
Invited Review
Palladium-Catalyzed Cross-Coupling Reactions of Cyclopropanols
David Rosa, Andrei Nikolaev, Nisha Nithiy and Arturo Orellana*
Synlett 2015, 26, 441
A review of the use of cyclopropanols in palladium-catalyzed C-C bond formation.
Invited Account
Nisha Nithiy and Arturo Orellana*
Organic Letters 2014, 16, 5854
We extend the range of oxidative addition partners that can be used in palladium-catalyzed cross-coupling reactions of cyclopropanols.
Andrei Nikolaev, Nisha Nithiy and Arturo Orellana*
Synlett 2014, 25, 2301
We develop a cascade reaction of cyclopropanols with bromoanilines.
Invited Contribution
Featured in the Organic Chemistry Portal
Highlighted in SYNFACTS
Liver X Receptors Preserve Renal Gromerular Integrity Under Normoglycaemia and in Diabetes in Mice
M. Patel, X. Wang, R. John, L. Magomedova, A. Rasheed, H. Santamaria, W. Wang, R. Tsai, L. Qiu, A. Orellana, M. Levi, C. Cummins*
Diabetologia, 2014, 57, 435
Collaboration with Carolyn Cummins - Leslie Dan School of Pharmacy, University of Toronto.
Carbon-Carbon Bond Formation Through Palladium Homoenolates
David Rosa, Nisha Nithiy and Arturo Orellana*
Synthesis, 2013, 45, 3199
We review all the methods known to generate palladium-homoenolates and their use in C-C cross-coupling.
Invited Review
Potassium Acetate
Arturo Orellana*
Encyclopedia of Reagents for Organic Synthesis 2013
Palladium-Catalyzed Cross-Coupling of Cyclopropanol-Derived Ketone Homoenolates with Aryl Bromides
David Rosa and Arturo Orellana*
Chemical Communications 2013, 49, 5420
We report the cross coupling of palladium homolates with aryl bromides. Judicious choice of ligands on palladium enables the coupling homoenolates with b-hydrides, extending the range of cyclopropanols that can be used in this reaction.
Featured in the Organic Chemistry Portal
Peter Liuni, Ekaterina Olkhov-Mitsel, Arturo Orellana and Derek Wilson*
Analytical Chemistry 2013, 85, 3758
Collaboration with Professor Derek Wilson - Center for Research in Mass Spectrometry
David Rosa, Andrei Chtchemelinine and Arturo Orellana*
Synthesis 2012, 44, 1885
We merge the palladium-catalyzed selective cleavage of benzocyclobutenols with decarboxylative allylation chemistry.
Invited Contribution
Palladium-Catalyzed Synthesis of Acyl Pyrroles from Aryl Iodides
Stephanie Ho, Ganna Bondarenko, David Rosa, Bojan Dragisic and Arturo Orellana*
The Journal of Organic Chemistry 2012, 77, 2008
We develop a new route to acyl pyrroles, which serve as surrogates to Weinreb amides. This work was primarily conducted by undergraduate thesis students.
David Rosa and Arturo Orellana*
Chemical Communications 2012, 48, 1922
This is one of the first reports using alkylpalladium intermediates in direct arylation chemistry.
Palladium-Catalyzed Selective Carboelimination and Cross-Coupling Reactions of Benzocyclobutenols.
Andrei Chtchemelinine, David Rosa and Arturo Orellana*
The Journal of Organic Chemistry 2011, 76, 9157
We report the first controlled cleavage and C-C bond forming reaction of benzocyclobutenols.
David Rosa and Arturo Orellana*
Organic Letters 2011, 13, 3648
We describe a very unusual, palladium-catalyzed rearrangement of tertiaryl allylic alcohols to ketones with rearrangement of the carbon framework.
Angelika Schweinitz, Andrei Chtchemelinine and Arturo Orellana*
Organic Letters 2011, 13, 232
We merge the palladium-catalyzed semipinacol rearrangement with direct arylation chemistry.
Palladium-Catalyzed Coupling of Cyclopropanols with Aryl Halides Under Mild Conditions.
David Rosa and Arturo Orellana*
Organic Letters 2011, 13, 110
We report the first cross-coupling reaction of cyclopropanols with aryl halides.